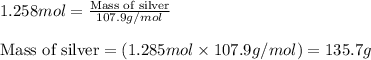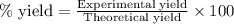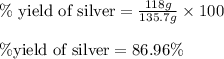Chemistry, 26.11.2019 00:31 agarcia24101993
When 40.0 g of copper are reacted with silver nitrate solution cu + 2 agno3 --> cu(no3)2 + 2 ag 118 g of silver are obtained. what is the percent yield of silver? molar mass of silver = 107.9 g, molar mass of copper = 63.55 g

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 12:00
Ageochemist examines a piece of metal that he found in the soil. he performs tests to identify the metal from its density, electrical conductivity, and melting point. which statement best describes his investigation? a. he is determining physical properties that are sufficient to identify the metal.b. he is determining chemical properties that are sufficient to identify the metal.c. he is determining physical properties that are insufficient to identify the metal.d. he is determining chemical properties that are insufficient to identify the metal.
Answers: 3


Chemistry, 22.06.2019 23:00
What is the average rate of the reaction between 10 and 20 s?
Answers: 1

Chemistry, 22.06.2019 23:50
Be sure to answer all parts. the following equilibrium constants were determined at 1123 k: c(s) + co2(g) ⇌ 2co(g) k'p = 1.30 × 1014 co(g) + cl2(g) ⇌ cocl2(g) k''p = 6.00 × 10−3 calculate the equilibrium constant at 1123 k for the reaction: c(s) + co2(g) + 2cl2(g) ⇌ 2cocl2(g) 4.68 × 10 9 (enter your answer in scientific notation.) write the equilibrium constant expression, kp:
Answers: 3
You know the right answer?
When 40.0 g of copper are reacted with silver nitrate solution cu + 2 agno3 --> cu(no3)2 + 2 ag...
Questions

Social Studies, 06.01.2021 09:50

English, 06.01.2021 09:50

Mathematics, 06.01.2021 09:50

Mathematics, 06.01.2021 09:50

Spanish, 06.01.2021 09:50

History, 06.01.2021 09:50

English, 06.01.2021 09:50


Mathematics, 06.01.2021 09:50






English, 06.01.2021 09:50


Mathematics, 06.01.2021 09:50

Mathematics, 06.01.2021 09:50


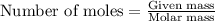 .....(1)
.....(1)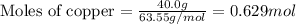
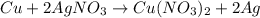
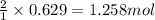 of silver
of silver