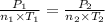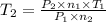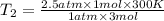Chemistry, 25.11.2019 21:31 ErrorNameTaken505
Aflask of fixed volume contains 1.0 mole of gaseous carbon dioxide and 88 g of solid carbon dioxide. the original pressure and temperature in the flask is 1.0 atm and 300. k. all of the solid carbon dioxide sublimes. the final pressure in the flask is 2.5 atm. what is the final temperature? assume the solid carbon dioxide takes up negligible volume. a. 150 kb. 200 kc. 250 kd. 300 ke. 400 k

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 03:00
Schrodinger and heisenberg developed an alternate theory about atomic nature that contradicted some of bohr's model of the atom. how do changes resulting from new technology and evidence affect the reputation of the atomic theory?
Answers: 1

Chemistry, 22.06.2019 06:30
This drawing shows a human body system. what is the primary function of this body system?
Answers: 3


Chemistry, 22.06.2019 23:00
What does a numerical subscript following an element in a chemical formula mean?
Answers: 1
You know the right answer?
Aflask of fixed volume contains 1.0 mole of gaseous carbon dioxide and 88 g of solid carbon dioxide....
Questions


Mathematics, 28.10.2019 23:31


History, 28.10.2019 23:31


English, 28.10.2019 23:31

Chemistry, 28.10.2019 23:31

Social Studies, 28.10.2019 23:31

Mathematics, 28.10.2019 23:31


Mathematics, 29.10.2019 00:31

Mathematics, 29.10.2019 00:31

Mathematics, 29.10.2019 00:31

Mathematics, 29.10.2019 00:31

Mathematics, 29.10.2019 00:31



Biology, 29.10.2019 00:31

Mathematics, 29.10.2019 00:31

Social Studies, 29.10.2019 00:31