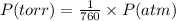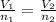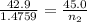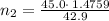Chemistry, 25.11.2019 21:31 ayoismeisalex
A42.9 l gas sample containing 1.50 moles of oxygen at 25.0 celcius exerts a pressure of 64.0.0 torr. the volume increases to 45.0 l when additional oxygen gas is added to the sample at constant temperature and pressure. how many moles have been added? what mass of gas was added?

Answers: 2


Another question on Chemistry



Chemistry, 23.06.2019 03:50
What is the equation fort the alkaline zinc/manganese dioxide cell. a) anode b)cathode c)overall equations.
Answers: 2

Chemistry, 23.06.2019 09:30
What is the force of an object when it landed(sitting in the ground)
Answers: 2
You know the right answer?
A42.9 l gas sample containing 1.50 moles of oxygen at 25.0 celcius exerts a pressure of 64.0.0 torr....
Questions

Chemistry, 13.12.2020 14:00

Physics, 13.12.2020 14:00

Computers and Technology, 13.12.2020 14:00

History, 13.12.2020 14:00



Spanish, 13.12.2020 14:00

Geography, 13.12.2020 14:00

English, 13.12.2020 14:00

English, 13.12.2020 14:00

English, 13.12.2020 14:00


Mathematics, 13.12.2020 14:00



Biology, 13.12.2020 14:00

Mathematics, 13.12.2020 14:00



English, 13.12.2020 14:00