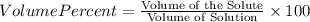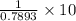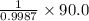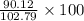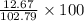Chemistry, 24.11.2019 12:31 Samanthas6365
Find both the volume percent of a solution that has 10.0 g of ethanol (d = 0.7893 g/ml) and 90.0 g of water (d = 0.9987 g/ml). assume volumes are additive.

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 22:30
Asample of neon occupies a volume of 375 ml at stp. what will be the volume of neon if the pressure is reduced to 90.0 kpa? a. 422 ml b. 422 l c. 333 ml d. 333 l
Answers: 2

Chemistry, 22.06.2019 06:30
Three cards with holes are arranged in a straight line. a light is shined through the first card’s hole and travels through all three cards. what does this tell you about light rays? a) that light is reflected b) that light is refractive c) that light travels in a straight line d) that light does not travel in a straight line
Answers: 1

Chemistry, 22.06.2019 09:50
Although respiratory organs vary across different organisms, they all contain respiratory surfaces that have a large surface area and are extremely thin. explain why having an extremely thin respiratory surface with a large surface area is advantageous for the process of gas exchange
Answers: 1

Chemistry, 22.06.2019 13:00
Is 9 correct? and can someone me with 10? it’s due tomorrow, you
Answers: 1
You know the right answer?
Find both the volume percent of a solution that has 10.0 g of ethanol (d = 0.7893 g/ml) and 90.0 g o...
Questions



History, 05.12.2020 09:00

English, 05.12.2020 09:00

Law, 05.12.2020 09:00


Physics, 05.12.2020 09:00



Mathematics, 05.12.2020 09:00



Mathematics, 05.12.2020 09:00

Physics, 05.12.2020 09:00



Mathematics, 05.12.2020 09:00

Chemistry, 05.12.2020 09:00


Mathematics, 05.12.2020 09:00