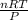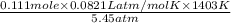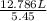Chemistry, 17.12.2019 04:31 khikhi1705
What would be the volume in liters of an ideal gas, if a 0.111 mole sample of the gas had a temperature of 1130 degrees celsius at a pressure of 5.45 atmospheres? (the ideal gas constant is 0.0821 l•atm/mol•k.)
0.43 liters
1.43 liters
1.89 liters
2.35 liters

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 20:30
9. write the chemical equation for the following word equations. include symbols for physical states in the equation. a. solid zinc sulfide + oxygen gas -> solid zinc oxide + sulfur dioxide gas b. aqueous hydrochloric acid + aqueous barium hydroxide -> aqueous barium chloride + water
Answers: 1

Chemistry, 21.06.2019 23:30
Start an single atom tab. observe the decay of polonium-211. after each decay, press the reset nucleus button to watch the process again. write a description of alpha decay for po-211
Answers: 2

Chemistry, 22.06.2019 00:00
Explain which group an element with the electron configuration 1s2 2s2 2p6 3s2 3p6 3d1 4s2 belongs to.
Answers: 3

Chemistry, 22.06.2019 00:00
Acurium-245 nucleus is hit with a neutron and changes as shown by the equation. complete the equation by filling in the missing parts. 52
Answers: 2
You know the right answer?
What would be the volume in liters of an ideal gas, if a 0.111 mole sample of the gas had a temperat...
Questions


Mathematics, 09.03.2021 19:00

Biology, 09.03.2021 19:00


Biology, 09.03.2021 19:00

Mathematics, 09.03.2021 19:00

Chemistry, 09.03.2021 19:00

Advanced Placement (AP), 09.03.2021 19:00



Mathematics, 09.03.2021 19:00

Mathematics, 09.03.2021 19:00

English, 09.03.2021 19:00

Mathematics, 09.03.2021 19:00


Mathematics, 09.03.2021 19:00



Mathematics, 09.03.2021 19:00