
A0.327-g sample of azulene (c10h8) is burned in a bomb calorimeter and the temperature increases from 25.20 °c to 27.60 °c. the calorimeter contains 1.17×103 g of water and the bomb has a heat capacity of 786 j/°c. based on this experiment, calculate δe for the combustion reaction per mole of azulene burned (kj/mol). c13h24o4(s) + 17 o2(g) 13 co2(g) + 12 h2o(l) e = kj/mol.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 09:50
What are four significant sources of ghgs that come from wostem washington?
Answers: 2

Chemistry, 22.06.2019 19:30
To calculate percent by mass, use the equation below: calculate the percent by mass of each element. %n = % %h = % %o = %
Answers: 3

Chemistry, 22.06.2019 23:30
The comparison of the number of atoms in a copper coin the size of a penny with the number of people on earth is made to illustrate which of the following? a. that atoms are indivisible b. that atoms are very small c. that atoms are very large d. that in a copper penny, there is one atom for every person on earth
Answers: 1

Chemistry, 23.06.2019 02:30
When the ionic compound nabr dissolves in water, br– ions are pulled into solution by the attraction between what two particles? a. the na+ and br– ions b. the na+ ion and the negative end of a water molecule c. the br– ion and the positive end of a water molecule d. the br– ion and the negative end of a water molecule
Answers: 1
You know the right answer?
A0.327-g sample of azulene (c10h8) is burned in a bomb calorimeter and the temperature increases fro...
Questions

Mathematics, 19.08.2019 08:20


Business, 19.08.2019 08:30


Mathematics, 19.08.2019 08:30

Mathematics, 19.08.2019 08:30

History, 19.08.2019 08:30

Mathematics, 19.08.2019 08:30


Mathematics, 19.08.2019 08:30

Chemistry, 19.08.2019 08:30

Mathematics, 19.08.2019 08:30




Mathematics, 19.08.2019 08:30



Mathematics, 19.08.2019 08:30

Biology, 19.08.2019 08:30

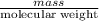
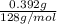
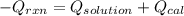
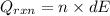
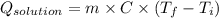
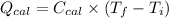
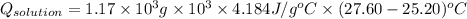
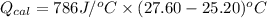
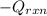 = (11748.67 + 1886.4) J
= (11748.67 + 1886.4) J
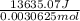
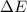 for the given combustion reaction per mole of azulene burned is 4452.26 kJ/mol.
for the given combustion reaction per mole of azulene burned is 4452.26 kJ/mol.

