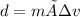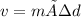Chemistry, 23.11.2019 06:31 sherry59Sherry59
Achemistry student needs 65.0g of diethylamine for an experiment. by consulting the crc handbook of chemistry and physics, the student discovers that the density of diethylamine is ·0.706gcm−3. calculate the volume of diethylamine the student should pour out.

Answers: 2


Another question on Chemistry


Chemistry, 22.06.2019 09:50
Although respiratory organs vary across different organisms, they all contain respiratory surfaces that have a large surface area and are extremely thin. explain why having an extremely thin respiratory surface with a large surface area is advantageous for the process of gas exchange
Answers: 1

Chemistry, 22.06.2019 19:00
How does a catalyst increase the speed of a reaction? a. the catalyst eliminates the activated complex stage, allowing products to form immediately. b. the catalyst lowers the energy level of the reactants, making it easier for them to react. c. the catalyst makes it easier for the activated complex to form, lowering the activation energy. d. the catalyst raises the energy level of the products, making the reaction finish sooner. reset next
Answers: 1

Chemistry, 23.06.2019 05:00
Which characteristics affect ocean water’s temperature? check all that apply. depth location mass salinity waves
Answers: 1
You know the right answer?
Achemistry student needs 65.0g of diethylamine for an experiment. by consulting the crc handbook of...
Questions

Mathematics, 28.11.2019 11:31



Mathematics, 28.11.2019 11:31

Physics, 28.11.2019 11:31


Mathematics, 28.11.2019 11:31



Mathematics, 28.11.2019 11:31

Biology, 28.11.2019 11:31


Physics, 28.11.2019 11:31

History, 28.11.2019 11:31

Chemistry, 28.11.2019 11:31