
Chemistry, 23.11.2019 04:31 Jenifermorales101
It turns out that the van der waals constant b equals four times the total volume actually occupied by the molecules of a mole of gas.
using this figure, calculate the fraction of the volume in a container actually occupied by ar atoms at 230 atm pressure and 0 c. assume b=0.0322 l/mol

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 06:00
Compare and contrast physical changes with chemical changes.
Answers: 3

Chemistry, 22.06.2019 07:20
After watching the video "zinc strip in copper nitrate solution", and reading the instructions, click on the link labeled "start" just below the drawing of the pencil tip. follow the direction to complete the 3x3 grid. answer the below questions for the portion of the activity in which sn(s) is placed in agno3(aq)
Answers: 1

Chemistry, 22.06.2019 23:00
How does the value of the equilibrium constant show that a reaction reaches equilibrium very quickly? (a) the equilibrium constant is large. (b) the equilibrium constant is small. (c) the equilibrium constant is zero. (d) the value of the equilibrium constant does not show how quickly a reaction comes to equilibrium.
Answers: 1

Chemistry, 23.06.2019 01:00
Which statement is true regarding the diagram of circle p? the sum of y and z must be 2x. the sum of y and z must be x. the difference of z and y must be 2x. the difference of z and y must be x
Answers: 1
You know the right answer?
It turns out that the van der waals constant b equals four times the total volume actually occupied...
Questions



Mathematics, 05.11.2020 18:50





English, 05.11.2020 18:50

Mathematics, 05.11.2020 18:50


Arts, 05.11.2020 18:50



Mathematics, 05.11.2020 18:50

Mathematics, 05.11.2020 18:50





History, 05.11.2020 18:50

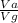
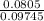 = 0.0826
= 0.0826

