Chemistry, 23.11.2019 04:31 lucilita2017
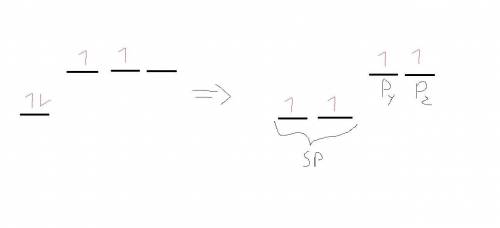
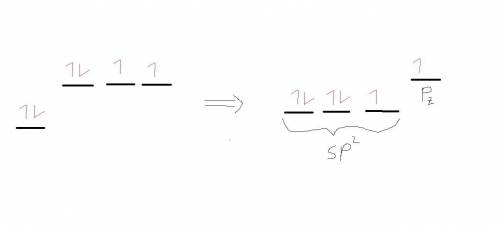
The lewis structure for co2 has a central the lewis structure for c o 2 has a central blank atom attached to blank atoms.
1. atom attached to the lewis structure for c o 2 has a central blank atom attached to blank atoms. atoms.
2. these atoms are held together by these atoms are held together by blank bonds. bonds.
3. carbon dioxide has a carbon dioxide has a blank electron geometry. electron geometry.
4. the carbon atom is the carbon atom is blank hybridized. hybridized.
5. carbon dioxide has two carbon dioxide has two blankπ bonds and two blankσ bonds.π bonds and two carbon dioxide has two blankπ bonds and two blankσ bonds.σ bonds.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 01:40
Which characteristic of water it form droplets? a. low specific heat b. nonpolar structure c. high surface tension d. ability to dissolve substances
Answers: 1

Chemistry, 22.06.2019 12:00
Ineed this asap part i: scientific method what is the difference between science and pseudoscience? what is the scientific method?
Answers: 2

Chemistry, 22.06.2019 15:30
How does a large body of water, such as the ocean, influence climate?
Answers: 1

Chemistry, 22.06.2019 16:30
4. a 20-kg child is tossed up into the air by her parent. the child is 2 meters off the ground traveling 5 m/s. circle one: ke / gpe / both show your work for finding the values of each type of energy the object has:
Answers: 1
You know the right answer?
The lewis structure for co2 has a central the lewis structure for c o 2 has a central blank atom att...
Questions



Mathematics, 20.05.2021 17:50


Law, 20.05.2021 17:50

Mathematics, 20.05.2021 17:50

Mathematics, 20.05.2021 17:50

Mathematics, 20.05.2021 17:50

Mathematics, 20.05.2021 17:50

Mathematics, 20.05.2021 17:50

Mathematics, 20.05.2021 17:50

English, 20.05.2021 17:50

History, 20.05.2021 17:50

Mathematics, 20.05.2021 17:50





Social Studies, 20.05.2021 17:50

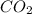 has a central Carbon atom attached to Oxygen atoms.
has a central Carbon atom attached to Oxygen atoms.