
Chemistry, 23.11.2019 03:31 talanna394
Consider the reaction directly below and answer parts a and b. c6h4(oh)2 (l) + h2o2 (l) à c6h4o2 (l) + 2 h2o (l)
(a) use the following information to calculate dh° for the above reaction. show all work. dh° c6h4(oh)2 (l) à c6h4o2 (l) + h2 (g) +177.4 kj h2 (g) + o2 (g) à h2o2 (l) -187.8 kj h2 (g) + o2 (g) à h2o (l) -285.8 kj
(b) based on your answer to part a, above, would heat be gained or lost by the surroundings as the reaction at the very top of the page occurred at standard conditions?
(c) how many kilojoules of heat are given off when 20.0 g of h2o (l) are produced at standard conditions according to the reaction at the very top of the page? show all work.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 23:00
When determining the shape of a molecule, it is important to draw a lewis dot structure first in order to see the total number a. electrons within the moleculeb. bonding and unshared pairs around central atomc. unshared pair within the molecule( i really need it )
Answers: 1


Chemistry, 22.06.2019 15:00
Many ionic compounds and a few highly polar covalent compounds are because they completely ionize in water to create a solution filled with charged ions that can conduct an electric current.
Answers: 1

Chemistry, 22.06.2019 17:10
Calculate the estimated density of each ball. use the formula d = m/v where d is the density, m is the mass, and v is the volume. record your calculations in table a of your student guide. given that the density of water is 1.0 g/cm3, make a prediction about whether each ball will float in water. record your prediction in table a. what is the estimated density of the table tennis ball? record your answer to the nearest hundredth
Answers: 2
You know the right answer?
Consider the reaction directly below and answer parts a and b. c6h4(oh)2 (l) + h2o2 (l) à c6h4o2 (l)...
Questions









Mathematics, 23.07.2019 13:00










Mathematics, 23.07.2019 13:00

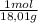 = 1,11 mol H₂O
= 1,11 mol H₂O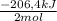 = -115kJ
= -115kJ

