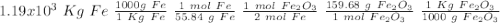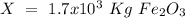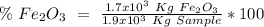Chemistry, 23.11.2019 00:31 mjweed3381
One of the reactions that occurs in a blast furnace, in which iron ore is converted to cast iron, is fe2o3 + 3co → 2fe + 3co2 suppose that 1.19 × 103 kg of fe is obtained from a 1.90 × 103 kg sample of fe2o3. assuming that the reaction goes to completion, what is the percent purity of fe2o3 in the original sample?

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 23:00
Ahypothrticalax type of ceramic material is known to have a density of 2.10 g/cm3 and a unit cell of cubic symmetry with a cell edge length of 0.57 nm. the atomic weights of the a and x elements are 28.5and 30.0 g/mol, respectively. on the basis of this information, which of the following crystal structures is (are) possible for this material: sodium chloride, cesium chloride, or zinc blende
Answers: 1

Chemistry, 22.06.2019 03:30
In saturated organic compounds, all the bonds between carbon atoms are called?
Answers: 1

Chemistry, 22.06.2019 09:00
At 300 mm hg, a gas has a volume of 380 l, what is the volume at standard pressure
Answers: 1

Chemistry, 22.06.2019 17:40
Areaction in which products can react to re-form reactants is
Answers: 1
You know the right answer?
One of the reactions that occurs in a blast furnace, in which iron ore is converted to cast iron, is...
Questions


Social Studies, 28.01.2020 13:38

Mathematics, 28.01.2020 13:38






Mathematics, 28.01.2020 13:38




Mathematics, 28.01.2020 13:38




Mathematics, 28.01.2020 13:38



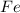 to grams of
to grams of 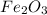 .
.