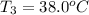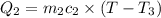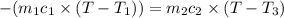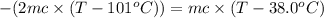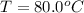Chemistry, 23.11.2019 00:31 sidallen05
One piece of copper jewelry at 101°c has twice the mass of another piece, which is at 38.0°c. both pieces are placed inside a calorimeter of negligible heat capacity. what is the final temperature inside the calorimeter (c of copper=0.387 j/g. k)?

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 18:30
Apure solvent has a vapor pressure the vapor pressure of a solution. a. equal to b. lower than c. higher than
Answers: 1

Chemistry, 22.06.2019 05:30
What type of reaction is shown below? check all that apply. 2h2o2 → 2h2o + o2 synthesis decomposition combustion
Answers: 3

Chemistry, 22.06.2019 06:00
Why is permeable soil best for plants that need a lot of drainage?
Answers: 1

Chemistry, 22.06.2019 16:30
How many grams of mgbr2 are needed to produce 75g or metal?
Answers: 1
You know the right answer?
One piece of copper jewelry at 101°c has twice the mass of another piece, which is at 38.0°c. both p...
Questions


Mathematics, 05.11.2019 22:31


Spanish, 05.11.2019 22:31



Mathematics, 05.11.2019 22:31






Mathematics, 05.11.2019 22:31

English, 05.11.2019 22:31

Physics, 05.11.2019 22:31


English, 05.11.2019 22:31

Mathematics, 05.11.2019 22:31


History, 05.11.2019 22:31

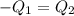
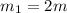
 = 0.387 J/g.K
= 0.387 J/g.K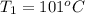
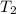 =T
=T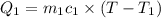
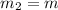
 = 0.387 J/g.K
= 0.387 J/g.K