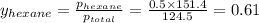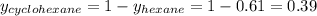Determine the mole fractions of each component in the vapor phase of the vapor in equilibrium with a 1: 1 molar ratio of hexane (c6h14) and cyclohexane (c6h12). the equilibrium vapor pressures of hexane and cyclohexane are equal to 151.4 torr and 97.6 torr respectively

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 06:00
Ethanol (c2h5oh) is produced from the fermentation of sucrose in the presence of enzymes. c12h22o11(aq) + h2o(g) 4 c2h5oh(l) + 4 co2(g) determine the theoretical yield and the percent yields of ethanol if 680. g sucrose undergoes fermentation and 326.5 g ethanol is obtained. theoretical _ g _ percent %
Answers: 1

Chemistry, 22.06.2019 08:30
Which part of earth’s surface receives the most direct rays from the sun? a) equator b) ocean c) poles d) mountains
Answers: 2


Chemistry, 22.06.2019 13:30
How many moles is 14.5 cm^3 of platinum? the density of platinum is 21.45 g/cm^3.
Answers: 1
You know the right answer?
Determine the mole fractions of each component in the vapor phase of the vapor in equilibrium with a...
Questions

History, 08.08.2019 18:10


Mathematics, 08.08.2019 18:10

Mathematics, 08.08.2019 18:10



Mathematics, 08.08.2019 18:10

Mathematics, 08.08.2019 18:10












Social Studies, 08.08.2019 18:10

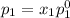 and
and 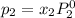
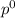 = pressure in the pure state
= pressure in the pure state
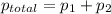
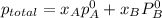
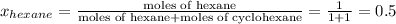 ,
, 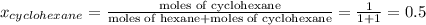 ,
, 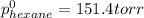
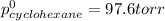
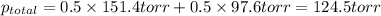
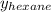 = mole fraction of hexane in vapor phase
= mole fraction of hexane in vapor phase