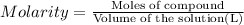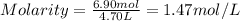Chemistry, 22.11.2019 23:31 shealwaysknows23
An aqueous magnesium chloride solution is made by dissolving 6.90 moles of mgcl 2 in sufficient water so that the final volume of the solution is 4.70 l . calculate the molarity of the mgcl 2 solution.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 22:30
What methods could you use to solubilize calcium carbonate
Answers: 1



Chemistry, 23.06.2019 11:20
Match each state of matter with the statement that best describes it.
Answers: 1
You know the right answer?
An aqueous magnesium chloride solution is made by dissolving 6.90 moles of mgcl 2 in sufficient wate...
Questions

















Computers and Technology, 02.12.2019 23:31