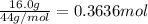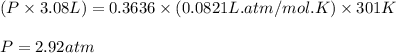Chemistry, 22.11.2019 23:31 oliviajewelwilliams
Cyclists sometimes use pressurized carbon dioxide inflators to inflate a bicycle tire in the event of a flat. these inflators use metal cartridges that contain 16.0 g of carbon dioxide.
at 301 k , to what pressure (in psi) can the carbon dioxide in the cartridge inflate a 3.08 l mountain bike tire?
(note: the gauge pressure is the difference between the total pressure and atmospheric pressure. in this case, assume that atmospheric pressure is 14.7 psi.)
express your answer to three significant figures with the appropriate units.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 08:00
An observation that requires measurement is called quantitative observable or qualitative
Answers: 1

Chemistry, 22.06.2019 12:00
Ican determine the molar mass of an element by looking on the under the atomic mass for the element. for example the molar mass of phosphorus is 30.974 grams/mole. avogadro’s number tells me the amount of representative particles in 1 mole of any substance. this means 12.011 gram sample of carbon and a 32.0 gram sample of sulfur have the same number of atoms.
Answers: 1


Chemistry, 22.06.2019 14:30
Select all of the statements which are true. electrons are located in shells or orbits around the atom. electrons orbit slowly around the atom. electrons travel in one flat path around the nucleus of an atom. the valence of an atom is determined by the number of electrons in the atom's outermost shell.
Answers: 1
You know the right answer?
Cyclists sometimes use pressurized carbon dioxide inflators to inflate a bicycle tire in the event o...
Questions

Mathematics, 31.07.2019 03:30


Mathematics, 31.07.2019 03:30



English, 31.07.2019 03:30


Mathematics, 31.07.2019 03:30

Mathematics, 31.07.2019 03:30




Chemistry, 31.07.2019 03:30

Biology, 31.07.2019 03:30




Chemistry, 31.07.2019 03:30