
Chemistry, 22.11.2019 21:31 samueltaye
Calculate δh and δstot when two copper blocks, each of mass 10.0 kg, one at 100°c and the other at 0°c, are placed in contact in an isolated container. the specific heat capacity of copper is 0.385 j k−1 g−1 and may be assumed constant over the temperature range involved.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 06:30
If 1.8 l of water is added to 2.5l of a 7.0 m koh solution, what is the molarity of the new solution
Answers: 1

Chemistry, 22.06.2019 12:30
How many grams of magnesium metal will react completely with 8.3 liters of 5.5m hcl? show all work
Answers: 1

Chemistry, 22.06.2019 12:30
Nebulae are enormous clouds in outer space. they are made mostly of hydrogen gas, helium gas, and dust. some nebulae glow brightly, while others do not. the stars that people see are huge, bright balls of glowing gas. they are made mostly of hydrogen and helium. which statement correctly describes other ways in which nebulae and stars are different? a. stars can form inside a nebula but a nebula can never be produced by any star. b. a star always has a higher density than a nebula. c. stars can never form inside a nebula but a nebula can be produced by any star. d. a nebula always has a higher density than a star.
Answers: 3

You know the right answer?
Calculate δh and δstot when two copper blocks, each of mass 10.0 kg, one at 100°c and the other at 0...
Questions

English, 20.04.2020 07:27

Biology, 20.04.2020 07:27



Mathematics, 20.04.2020 07:27

English, 20.04.2020 07:27

English, 20.04.2020 07:27


Chemistry, 20.04.2020 07:28

Mathematics, 20.04.2020 07:28

Mathematics, 20.04.2020 07:28

Biology, 20.04.2020 07:28

Chemistry, 20.04.2020 07:28


English, 20.04.2020 07:28


English, 20.04.2020 07:28



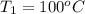 = (100 + 273) K = 373 K
= (100 + 273) K = 373 K 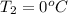 = (0 + 273) K = 273 K
= (0 + 273) K = 273 K
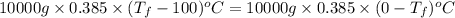
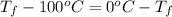
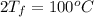
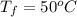
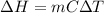
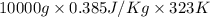
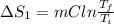
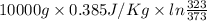

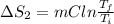

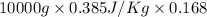
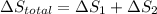
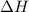 is 1243.5 kJ and
is 1243.5 kJ and 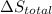 is 93.37 J/K.
is 93.37 J/K.

