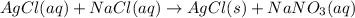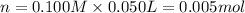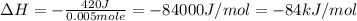Chemistry, 22.11.2019 19:31 jadabecute3739
 + nacl(aq) \rightarrow agcl(s)\ +\ nano _3(aq)) in an experiment a student mixes a 50.0 ml sample of 0.100 m agno₃ (aq) with a 50.0 ml sample of 0.100 m nacl(aq) at 20.0°c in a coffee-cup calorimeter. which of the following is the enthalpy change of the precipitation reaction represented above if the final temperature of the mixture is 21.0°c? (assume that the total mass of the mixture is 100. g and that the specific heat capacity of the mixture is 4.2 j/(g.° (a) -84 kj/mol, (b) -0.42 kj/mol (c) 0.42 kj/molu (d) 84 kj/molpx
in an experiment a student mixes a 50.0 ml sample of 0.100 m agno₃ (aq) with a 50.0 ml sample of 0.100 m nacl(aq) at 20.0°c in a coffee-cup calorimeter. which of the following is the enthalpy change of the precipitation reaction represented above if the final temperature of the mixture is 21.0°c? (assume that the total mass of the mixture is 100. g and that the specific heat capacity of the mixture is 4.2 j/(g.° (a) -84 kj/mol, (b) -0.42 kj/mol (c) 0.42 kj/molu (d) 84 kj/molpx

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 12:50
Which features are shown in the image? check all that apply. folds o anticlines o synclines o normal faults ostrike-slip faults
Answers: 1

Chemistry, 22.06.2019 00:00
Select the correct answer. which statement is true about a polarized object? o a. it gains electrons and becomes negatively charged. ob. it gains protons and becomes positively charged. oc. the number of positive and negative charges can be the same. od. it has to be a metal. o e. there is no change in the distribution of the charge in the object. reset next what
Answers: 3

Chemistry, 22.06.2019 13:00
If two objects at different te,peraure are in contact with each other what happens to their temperature
Answers: 1

Chemistry, 22.06.2019 23:00
Consider the reaction: 2al(s) + fe2o3(s) → al2o3(s) + 2fe(s) the δhf for fe2o3(s) = -824.3 kj/mole. the δhf for al2o3(s) = -1675.7 kj/mole. finish the equation. δhrxn = [(1)( kj/mole) + (2)( kj/mole)] - [(1)( kj/mole) + (2) ( kj/mole)]
Answers: 1
You know the right answer?
[tex]agno _3(aq) + nacl(aq) \rightarrow agcl(s)\ +\ nano _3(aq)[/tex]in an experiment a student mixe...
Questions



Mathematics, 26.04.2021 02:50

Mathematics, 26.04.2021 02:50








Physics, 26.04.2021 02:50


Business, 26.04.2021 02:50

Business, 26.04.2021 02:50

English, 26.04.2021 02:50



Mathematics, 26.04.2021 02:50

Mathematics, 26.04.2021 02:50

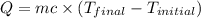
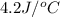
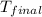 = final temperature =
= final temperature = 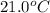
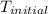 = initial temperature =
= initial temperature = 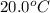
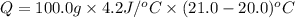
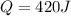
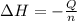
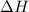 = enthalpy change = ?
= enthalpy change = ?