Answers: 3


Another question on Chemistry


Chemistry, 22.06.2019 19:20
Anyone who's in connections academy chemistry b have the factors that affect the rate of a reaction portfolio already done?
Answers: 3

Chemistry, 22.06.2019 21:00
Write a balanced equation showing the formation of copper (ii) nitrite from its elements
Answers: 1

You know the right answer?
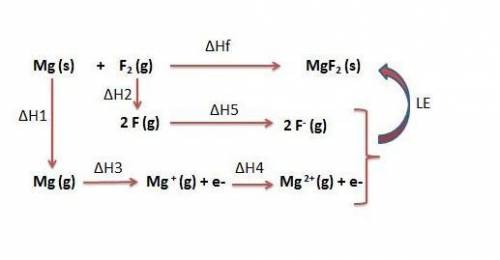
Use the following to calculate h°lattice of mgf2. mg(s) mg(g) h° = 148 kj f2(g) 2 f(g) h° = 159 kj m...
Questions


Geography, 13.05.2021 16:40

Computers and Technology, 13.05.2021 16:40

English, 13.05.2021 16:40

Mathematics, 13.05.2021 16:40



History, 13.05.2021 16:40




Mathematics, 13.05.2021 16:40

History, 13.05.2021 16:40

Mathematics, 13.05.2021 16:40

Mathematics, 13.05.2021 16:40

Mathematics, 13.05.2021 16:40

Health, 13.05.2021 16:40

Mathematics, 13.05.2021 16:40

Mathematics, 13.05.2021 16:40

Arts, 13.05.2021 16:40