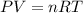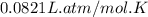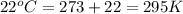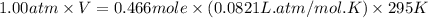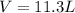Automobile airbags contain solid sodium azide, nan3, that reacts to produce nitrogen gas when heated, thus inflating the bag. 2nan3(s) > 2na(s) + 3n2(g) calculate the value of w (work) for the following system if 20.2 g of nan3 reacts completely at 1.00 atm and 22 degrees

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 19:10
When will le chatelier's principle come into effect? at the beginning of a reaction, when there are only reactants when a reaction has reached chemical equilibrium when a catalyst is added to a reaction mixture when a reaction is occurring but not yet at equilibrium
Answers: 3

Chemistry, 21.06.2019 22:30
Each of the following compounds contains a metal that can exhibit more than one ionic charge. provide systematic names for each of these compounds. (a) cr(clo3)6 (b) mo(cn)6 (c) cr2(so3)3 (d) v(clo2)2 (e) v(cn)5 (f) os(clo2)4
Answers: 3

Chemistry, 22.06.2019 03:40
In an effort to address concerns about global warming, a power plant in portland,oregon is designed to take all of its exhaust gases from its boilers and recycle the co2 using the solvay process to make sodium hydrogen carbonate. the reaction is shown below. nh3(g) + h2o(l) + co2(g) + nacl(aq) → nahco3(aq) + nh4cl(aq) how many liters each of nh3 and co2 (both at stp) would be consumed to produce 3.00 kg of sodium bicarbonate? the volume of both nh3 and co2 would be
Answers: 1

Chemistry, 22.06.2019 07:00
6what is the importance of water on earth? a) it keeps the top layer of the geosphere cool b) it allows life to exist c) it provides ice at the poles d) it creates earth's blue color from space
Answers: 2
You know the right answer?
Automobile airbags contain solid sodium azide, nan3, that reacts to produce nitrogen gas when heated...
Questions


Mathematics, 09.03.2021 17:20




Mathematics, 09.03.2021 17:20


Mathematics, 09.03.2021 17:20

Mathematics, 09.03.2021 17:20


English, 09.03.2021 17:20


Chemistry, 09.03.2021 17:20


Mathematics, 09.03.2021 17:20

Mathematics, 09.03.2021 17:20




Social Studies, 09.03.2021 17:20

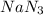
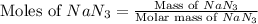
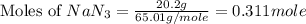
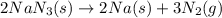
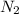
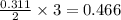 moles of
moles of