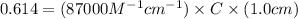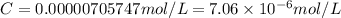Chemistry, 22.11.2019 04:31 wutwut2261
The absorbance of a crystal violet solution in water (density is 1.00 g/ml) is measured in a 1.0 cm cuvette. the absorbance is 0.614 and the molar absorptivity coefficient is the molecular weight of crystal violet is 407.98 g/mol. what is the molar concentration of the solution?

Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 10:00
Ill give brainiestif one neutron initiates a fission event that produces two neutrons in the products, how many new reactions can now be initiated? if each of the neutrons produced in the first fission event then initiates a fission event that produces one neutron in the products, how many new reactions can now be initiated by each neutron? how many neutrons in total were produced by the two fission events described?
Answers: 2

Chemistry, 22.06.2019 18:30
The table lists the lattice energies of some compounds.compoundlattice energy (kj/mol)lif –1,036licl –853naf –923kf –821nacl –786which statement about crystal lattice energy is best supported by the information in the table? the lattice energy increases as cations get smaller, as shown by lif and kf.the lattice energy increases as the cations get larger, as shown by lif and licl.the lattice energy decreases as cations get smaller, as shown by nacl and naf.the lattice energy decreases as the cations get smaller, as shown by naf and kf.
Answers: 3

Chemistry, 22.06.2019 19:30
If 16.00g of hydrogen gas reacts with 126.73g of oxygen, how many grams of water are yielded? (both reactants are completely consumed in the reaction.)
Answers: 2
You know the right answer?
The absorbance of a crystal violet solution in water (density is 1.00 g/ml) is measured in a 1.0 cm...
Questions


Business, 19.10.2019 22:30


Mathematics, 19.10.2019 22:30


Biology, 19.10.2019 22:30


Mathematics, 19.10.2019 22:30

English, 19.10.2019 22:30

Mathematics, 19.10.2019 22:30


History, 19.10.2019 22:30

English, 19.10.2019 22:30




History, 19.10.2019 22:30


Mathematics, 19.10.2019 22:30

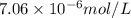

 = molar absorptivity coefficient =
= molar absorptivity coefficient = 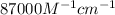 (assume)
(assume)