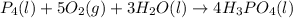Phosphoric acid, which is commonly used as rust inhibitor, food additive and etching agent for dental and orthopedic use, can be synthesized using a two-step thermal process. in the first step, phosphorus and oxygen react to form diphosphorus pentoxide: p4(l)+5o2(g-2 p20s(g) in the second step, diphosphorus pentoxide and water react to form phosphoric acld p20(9)+3 h200 2h, po40) write the net chemical equation for the production of phosphoric acid from phosphorus, oxygen and water. be sure your equation is balanced.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 04:30
Long term exposure to waves can cause sunburns and skin cancer. a) visible b) infrared c) gamma rays d) ultraviole
Answers: 1


Chemistry, 22.06.2019 13:10
What type of interaction occurs between the r groups of valine and isoleucine in a tertiary structure? view available hint(s) what type of interaction occurs between the r groups of valine and isoleucine in a tertiary structure? salt bridge disulfide bridge hydrogen bond hydrophobic interaction
Answers: 1

You know the right answer?
Phosphoric acid, which is commonly used as rust inhibitor, food additive and etching agent for denta...
Questions

Mathematics, 23.11.2020 20:30



English, 23.11.2020 20:30

Mathematics, 23.11.2020 20:30


English, 23.11.2020 20:30


Chemistry, 23.11.2020 20:40


Mathematics, 23.11.2020 20:40

Mathematics, 23.11.2020 20:40

Mathematics, 23.11.2020 20:40

Mathematics, 23.11.2020 20:40

Physics, 23.11.2020 20:40


Mathematics, 23.11.2020 20:40

Social Studies, 23.11.2020 20:40


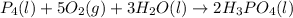
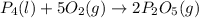 ......(1)
......(1)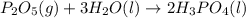 .......(2)
.......(2)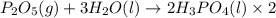
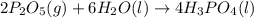 .......(3)
.......(3)