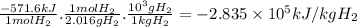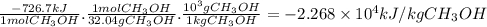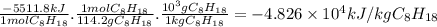Chemistry, 22.11.2019 03:31 playaajosh
Hydrogen and methanol have both been proposed as alternatives to hydrocarbon fuels. write balanced reactions for the complete combustion of hydrogen and methanol (identify phases) and use standard enthalpies of formation to calculate the amount of heat released per kilogram of the fuel (kj/kg). which fuel contains the most energy in the least mass? how does the energy of these fuels compare to that of octane (c8h18) (amount of heat released by octane (kj/

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 05:50
What are the 4 phases of matter in order of increasing engery content?
Answers: 2

Chemistry, 22.06.2019 13:00
Imagine that you push on a large rock. at what point does your effort change the rock’s mechanical energy?
Answers: 1

Chemistry, 22.06.2019 13:50
Abeaker with 2.00×102 ml of an acetic acid buffer with a ph of 5.000 is sitting on a benchtop. the total molarity of acid and conjugate base in this buffer is 0.100 m. a student adds 4.70 ml of a 0.360 m hcl solution to the beaker. how much will the ph change? the pka of acetic acid is 4.740.
Answers: 1

Chemistry, 22.06.2019 16:50
Which element is least likely to undergo a chemical reaction
Answers: 3
You know the right answer?
Hydrogen and methanol have both been proposed as alternatives to hydrocarbon fuels. write balanced r...
Questions



Mathematics, 16.12.2020 16:40


Mathematics, 16.12.2020 16:40

Mathematics, 16.12.2020 16:40




Biology, 16.12.2020 16:40




Biology, 16.12.2020 16:40


Mathematics, 16.12.2020 16:40


English, 16.12.2020 16:40