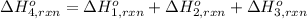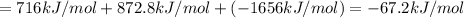Chemistry, 22.11.2019 03:31 CyberSongWriter
Using the following information and the fact that the average c―h bond enthalpy is 414 kj/mol, estimate the standard enthalpy of formation of methane (ch4). c(s) → c(g) δh o rxn = 716 kj/mol 2h2(g) → 4h(g) δh o rxn = 872.8 kj/mol

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 17:30
This is a mixture that has the same composition throughout.
Answers: 1

Chemistry, 22.06.2019 03:30
In this chemical reaction, 325 grams of barium (ba) react completely. how many moles of lithium (li) are produced?
Answers: 1

Chemistry, 22.06.2019 19:50
Identify the lewis base in this balanced equation: fe3+ h2o fe(h2o)63+
Answers: 1

Chemistry, 23.06.2019 00:30
What is the chemical formula of magnesium bromide? a. mgbr2 b. mgbr c. mg2br2 d. mg2br
Answers: 3
You know the right answer?
Using the following information and the fact that the average c―h bond enthalpy is 414 kj/mol, estim...
Questions



History, 15.10.2019 18:00











Physics, 15.10.2019 18:00

Computers and Technology, 15.10.2019 18:00

Chemistry, 15.10.2019 18:00

Mathematics, 15.10.2019 18:00

Mathematics, 15.10.2019 18:00


Mathematics, 15.10.2019 18:00

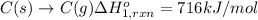 ...[1]
...[1]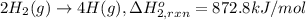 ...[2]
...[2]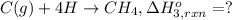 ...[3]
...[3]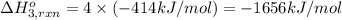
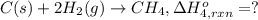 ...[4]
...[4]