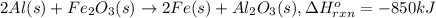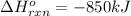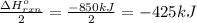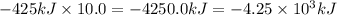Chemistry, 22.11.2019 03:31 lanashanabJHsbd1099
Calculate the enthalpy change for the thermite reaction: 2al(s)+fe2o3(s)→2fe(s)+al2o3(s), δh∘rxn=−850 kj when 10.0 mol of al undergoes the reaction with a stoichiometrically equivalent amount of fe2o3. express your answer to three significant figures and include the appropriate units.

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 17:10
There are 6.022 x 10^23 atoms of hg in 1 mole of hg. the number of atoms in 4.5 moles of hg can be found by multiplying 4.5 by 6.022 x 10^23 a. 2.7 x 10^24 b. 27 x 10^23 c. 2.71 x10^24 d. 27.099 x 10^23
Answers: 3

Chemistry, 22.06.2019 06:10
How many moles of gas are present if p=11 atm, v=12l, t=185k?
Answers: 1

Chemistry, 22.06.2019 13:00
Jose and eric were given four samples in lab. the results of their analysis are shown in the table. based on the data they collected, which sample is most likely a metal?
Answers: 1

You know the right answer?
Calculate the enthalpy change for the thermite reaction: 2al(s)+fe2o3(s)→2fe(s)+al2o3(s), δh∘rxn=−8...
Questions

Mathematics, 11.12.2020 05:40




Mathematics, 11.12.2020 05:50



Mathematics, 11.12.2020 05:50

Social Studies, 11.12.2020 05:50


Mathematics, 11.12.2020 05:50


Mathematics, 11.12.2020 05:50


Mathematics, 11.12.2020 05:50


English, 11.12.2020 05:50


Mathematics, 11.12.2020 05:50

Mathematics, 11.12.2020 05:50

 .
.