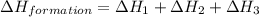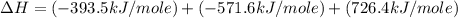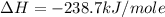Calculate the standard enthalpy of formation of liquid methanol, ch3oh(l), using the following information: c(graphite) + o2 --> co2(g) latex: \deltaδh° = –393.5 kj/mol h2(g) + (1/2)o2 --> h2o(l) latex: \deltaδh° = –285.8 kj/mol ch3oh(l) + (3/2)o2(g) --> co2(g) + 2h2o(l) latex: \deltaδh° = –726.4 kj/mol

Answers: 3


Another question on Chemistry


Chemistry, 22.06.2019 15:30
Why does earth rotate? because earth is formed from cold gases collapsing due to gravity because the matter in the nebula that formed earth was spinning because earth forms more than 99% of the mass of the solar system because the hydrogen atoms inside the nebula fused to form helium
Answers: 1

Chemistry, 22.06.2019 21:50
Given the data below for the reaction, 2 a + 2 b + 4 c => d + e + 3 f, the reaction is fill in the [ ] order in a, fill in the [ ] order in b, fill in the [ ] order in c and fill in the [ ] order overall. (use the words "first, second, third, fourth" to fill each blank)experimentinitial conc of a, mol/l initial conc of b, mol/l initial conc of c, mol/l initial rate, mol/l.s1 0.1 0.1 0.2 2 x 10-32 0.2 0.3 0.2 6 x 10-33 0.3 0.1 0.2 2 x 10-34 0.4 0.3 0.4 1.2 x 10-2
Answers: 2

Chemistry, 22.06.2019 22:30
What is a number added in front of a formula in order to balance the equation
Answers: 1
You know the right answer?
Calculate the standard enthalpy of formation of liquid methanol, ch3oh(l), using the following infor...
Questions


History, 17.07.2019 04:30


Biology, 17.07.2019 04:30

Mathematics, 17.07.2019 04:30


Mathematics, 17.07.2019 04:30




History, 17.07.2019 04:30


Arts, 17.07.2019 04:30

Social Studies, 17.07.2019 04:30






English, 17.07.2019 04:30

 will be,
will be,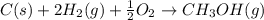
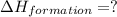
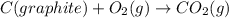
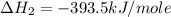
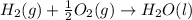
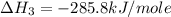
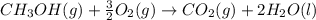
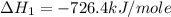
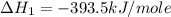
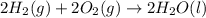
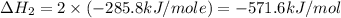
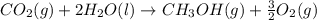
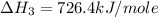
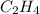 will be,
will be,