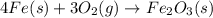Answers: 1


Another question on Chemistry


Chemistry, 23.06.2019 02:20
In a chemical reaction, the final amount of the products is determined by the a. universal gas law b. law of definite proportions c. air pressure d. temperature e. none of the above me
Answers: 2

Chemistry, 23.06.2019 05:40
Why is any chemical reaction always balanced? give reasons and explain the easiest way to solve the balancing problems in chemical equations with stoichiometric coefficients upto 20 as hit and trial doesn't always work. give full reasoning
Answers: 1

Chemistry, 23.06.2019 06:00
Amanda pushes a box across the room with a force of 30 n. it accelerates at 5 m/s/s. what is the mass of the box? * 6 kg 1.16 kg 30 kg 5kg
Answers: 2
You know the right answer?
The reaction, 4fe (s) + 3o2 (g) à 2 fe2o3 (s), is the rusting of iron. what occurs as the reaction p...
Questions


Engineering, 18.06.2021 07:40

Mathematics, 18.06.2021 07:40

Mathematics, 18.06.2021 07:40

Health, 18.06.2021 07:40




Law, 18.06.2021 07:40

Biology, 18.06.2021 07:40

Mathematics, 18.06.2021 07:40

Mathematics, 18.06.2021 07:40




English, 18.06.2021 07:40


English, 18.06.2021 07:50

English, 18.06.2021 07:50

Physics, 18.06.2021 07:50