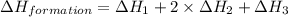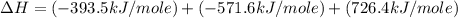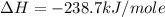Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 15:00
If a planet rotates 360 degrees during a 24 hour time period, what does that tell us about the planet? a. the middle of the planet is in darkness b. the seasons on the planet vary every day. c. the planet runs on a 12-hour time clock. d. the temperature on the planet varies daily.
Answers: 1

Chemistry, 21.06.2019 21:00
Of the groups of elements below, which are most likely to gain electrons to become anions? a. alkali metal b. boron group c. halogen d. transition metal
Answers: 2

Chemistry, 22.06.2019 10:30
Balance and in which category does it fit in? single or double displacement or synthesis or decomposition? (a) k2 o → k + o2 (b) na + i2 → nai (c) cu(no3 )2 + naoh → cu(oh)2 + nano3 (d) kclo3 → kcl + o2 (e) ca(no3 )2 + hbr → cabr2 + hno3 (f) sn(oh)2 → sno + h2 o (g) p4 + n2 o → p4 o6 + n2 (h) fe + al2 (so4 )3 → feso4 + al (i) alcl3 + na2 co3 → al2 (co3 )3 + nacl (j) c3 h6 + o2 → co2 + h2 o
Answers: 1

You know the right answer?
Given the following heats of combustion. ch3oh(l) + 3/2 o2(g) co2(g) + 2 h2o(l) δh°rxn = -726.4 kj c...
Questions

Mathematics, 04.12.2019 21:31

History, 04.12.2019 21:31

Mathematics, 04.12.2019 21:31


Mathematics, 04.12.2019 21:31





Engineering, 04.12.2019 21:31

Physics, 04.12.2019 21:31


Chemistry, 04.12.2019 21:31


Biology, 04.12.2019 21:31



Advanced Placement (AP), 04.12.2019 21:31


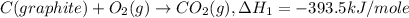 ..[1]
..[1] ..[2]
..[2]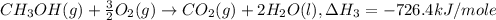 ..[3]
..[3] ..[1]
..[1] ..[2]
..[2]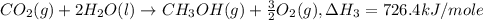 [3]
[3] will be,
will be,