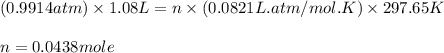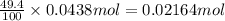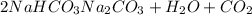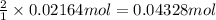You determine the volume of your plastic bag (simulated human stomach) is 1.08 l. how many grams of nahco3 (s) are required to fill this container given a 49.4% co2 recovery, assuming the other contents in the bag take up a negligible volume compared to the gas. the temperature of the room is 24.5 °c and the atmospheric pressure is 753.5 mmhg.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 08:30
What is the independent variable in this investigation? mass volume sample number substance density
Answers: 3

Chemistry, 22.06.2019 10:50
8) a mixture of he, ne and ar has a pressure of 7.85 atm. if the ne has a mole fraction of 0.47 and 8) ar has a mole fraction of 0.23, what is the pressure of he? a) 4.2 atm b) 3.7 atm c) 5.5 atm d) 2.4 atm e) 1.8 atm
Answers: 1

Chemistry, 22.06.2019 13:30
What does the xylem do? stores the glucose captures the sunlight absorbs oxygen into the leaf carries water from the roots to the leaves
Answers: 1

Chemistry, 22.06.2019 19:00
How does a catalyst increase the speed of a reaction? a. the catalyst eliminates the activated complex stage, allowing products to form immediately. b. the catalyst lowers the energy level of the reactants, making it easier for them to react. c. the catalyst makes it easier for the activated complex to form, lowering the activation energy. d. the catalyst raises the energy level of the products, making the reaction finish sooner. reset next
Answers: 1
You know the right answer?
You determine the volume of your plastic bag (simulated human stomach) is 1.08 l. how many grams of...
Questions

Mathematics, 18.03.2021 21:10


Social Studies, 18.03.2021 21:10

Business, 18.03.2021 21:10


Mathematics, 18.03.2021 21:10



Chemistry, 18.03.2021 21:10

Biology, 18.03.2021 21:10

Mathematics, 18.03.2021 21:10





Mathematics, 18.03.2021 21:10

Mathematics, 18.03.2021 21:10


Biology, 18.03.2021 21:10

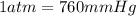 )
)