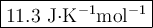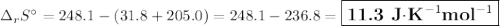The value of δs° for the oxidation of solid elemental sulfur to gaseous sulfur dioxide, s (s, rhombic) + o2 (g) → so2 (g) is j/k⋅mol. the value of s° for the oxidation of solid elemental sulfur to gaseous sulfur dioxide, s (s, rhombic) + o2 (g) so2 (g) is j/kmol. +248.5 +485.4 +11.6 -11.6 -248.5

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 18:50
Which term refers to a property that depends only on the amount of a substance? ©@
Answers: 2

Chemistry, 22.06.2019 22:00
Choose all the answers that apply. fluorine (f) has an atomic number of 9 and an atomic weight of 18.99. fluorine has a. 9 protons b. 10 neutrons c. 18 electrons d. an atomic mass of 19 e. at least one isotope
Answers: 1


Chemistry, 22.06.2019 23:30
Why do oxygen have a strong attractive force for electrons
Answers: 2
You know the right answer?
The value of δs° for the oxidation of solid elemental sulfur to gaseous sulfur dioxide, s (s, rhombi...
Questions

Mathematics, 25.09.2019 18:00

Social Studies, 25.09.2019 18:00

History, 25.09.2019 18:00

Mathematics, 25.09.2019 18:00


History, 25.09.2019 18:00


Health, 25.09.2019 18:00



Biology, 25.09.2019 18:00

Biology, 25.09.2019 18:00



Social Studies, 25.09.2019 18:00

Mathematics, 25.09.2019 18:00



Business, 25.09.2019 18:00

Spanish, 25.09.2019 18:00