
Chemistry, 21.11.2019 23:31 katielloyd
A1.800 g sample of octane, c8h18, is burned a calorimeter whose total heat capacity is 12.66 kj/°c. if the temperature of the calorimeter increased from 22.36 °c to 28.78 °c, then what is the δh for the combustion of one mole of octane? do not add the unit in the answer.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 03:30
Explain why pure hydrogen cyanide does not conduct electricity, but become a conductor when it is dissolved in water? (at room temp, pure hcn exists as a volatile liquid)
Answers: 1

Chemistry, 22.06.2019 07:00
How many moles are in 7.2 x 10^23 carbon molecules? (*round to the nearest hundredth and include the unit "mol c" after your number) question 6 options:
Answers: 2


Chemistry, 22.06.2019 19:30
Which one of the following substances would be the most soluble in ccl4? na2so4 h2o ch3ch2ch2ch2oh c4h10 hi
Answers: 1
You know the right answer?
A1.800 g sample of octane, c8h18, is burned a calorimeter whose total heat capacity is 12.66 kj/°c....
Questions

Mathematics, 08.12.2020 17:50


History, 08.12.2020 17:50

Biology, 08.12.2020 17:50




History, 08.12.2020 17:50

Mathematics, 08.12.2020 17:50


English, 08.12.2020 17:50

Mathematics, 08.12.2020 17:50

Mathematics, 08.12.2020 17:50

Mathematics, 08.12.2020 17:50

Arts, 08.12.2020 17:50

Mathematics, 08.12.2020 17:50

Chemistry, 08.12.2020 17:50


History, 08.12.2020 17:50

Mathematics, 08.12.2020 17:50

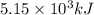
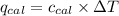
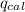 = Heat gained by bomb calorimeter
= Heat gained by bomb calorimeter 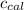 =Heat capacity of bomb calorimeter=12.66 kJ/°C
=Heat capacity of bomb calorimeter=12.66 kJ/°C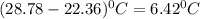
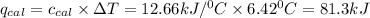
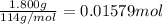
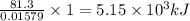 of heat
of heat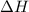 for the combustion of one mole of octane is
for the combustion of one mole of octane is 

