Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 14:00
Classify each statement about effective nuclear charge, zeff, as true or false.
Answers: 2

Chemistry, 21.06.2019 23:00
Esign techniques and materials that reduce the negative environmental impact of a structure are referred to as
Answers: 3

Chemistry, 22.06.2019 04:50
Compare the equilibrium constants for the systems shown in the table. which favors products the most? which favors products the least? rank these systems in order from most to least in terms of favoring products rather than reactants. d > b > a > c c > a > b > d b > c > d > a a > d > c > b
Answers: 1

Chemistry, 22.06.2019 07:00
Indicate whether the specified alkyl halides will form primarily substitution products, only elimination products, both substitution and elimination products, or no products when they react with sodium methoxide. 1-bromobutane 1-bromo-2-methylpropane 2-bromobutane 2-bromo-2-methylpropane
Answers: 2
You know the right answer?
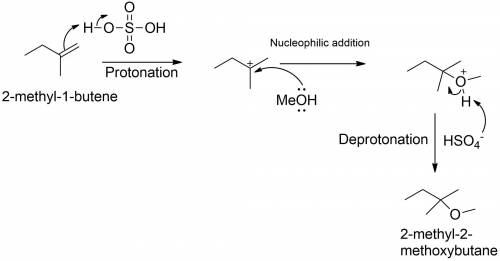
Treating 2-methyl-1-butene with methanol in the presence of sulfuric acid gives 2-methoxy-2-methylbu...
Questions


English, 18.08.2019 19:00

History, 18.08.2019 19:00

History, 18.08.2019 19:00


History, 18.08.2019 19:00



Mathematics, 18.08.2019 19:00

Mathematics, 18.08.2019 19:00

Mathematics, 18.08.2019 19:00


Mathematics, 18.08.2019 19:00

Mathematics, 18.08.2019 19:00




Mathematics, 18.08.2019 19:00

Mathematics, 18.08.2019 19:00