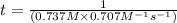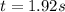Chemistry, 21.11.2019 21:31 treytonmesser
The reaction a → products was found to be second order order and have a rate constant, k, of 0.707 m-1 s-1. if the initial concentration of the reaction was 0.737 m, what is the half life for the reaction?

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 07:30
The volume of helium in a blimp is 6.28 x 10^9 millimeters. the density of helium in the blimp is .1786 kilogram/meter^3. find the mass of the helium in the blimp.
Answers: 1

Chemistry, 22.06.2019 10:30
When the speed of the bottle is 2 m/s, the average maximum height of the beanbag is m.
Answers: 2

Chemistry, 22.06.2019 17:30
A650 ml sodium bromine solution has a bromide ion concentration of 0.245 m. what is the mass (g) of sodium bromide in solution? a) 103.b)0.00155.c)16400.d) 16.4.e) 0.159
Answers: 2

Chemistry, 22.06.2019 22:30
Vi limitens. vastery test select the correct answer. which statement explains why large atoms are more reactive than small atoms? a. large atoms have valence electrons farther from the nucleus and lose them more readily. b. large atoms have greater ionization energy, which they can utilize during a reaction. c. large atoms have a greater number of electrons that they can lose during a reaction. d. large atoms have more energy levels, so they have more energy to pass on in a reaction. reset next
Answers: 3
You know the right answer?
The reaction a → products was found to be second order order and have a rate constant, k, of 0.707 m...
Questions




Social Studies, 12.08.2020 19:01




Mathematics, 12.08.2020 19:01






Engineering, 12.08.2020 19:01

Mathematics, 12.08.2020 19:01

Mathematics, 12.08.2020 19:01




![\frac{1}{[A]_{t}}=kt+\frac{1}{[A]_{0}}](/tpl/images/0385/0648/16aaf.png)
![[A]_{t}](/tpl/images/0385/0648/c37dd.png) is concentration of A after "t" time and
is concentration of A after "t" time and ![[A]_{0}](/tpl/images/0385/0648/48818.png) is initial concentration of A
is initial concentration of A![[A]_{t}=\frac{[A]_{0}}{2}](/tpl/images/0385/0648/2b76d.png)
![[A]_{0}=0.737M](/tpl/images/0385/0648/c3ec8.png) and
and 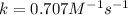
![\frac{1}{\frac{[A]_{0}}{2}}=(0.707M^{-1}s^{-1}\times t)+\frac{1}{[A]_{0}}](/tpl/images/0385/0648/7be48.png)
![\frac{1}{[A]_{0}}=0.707M^{-1}s^{-1}\times t](/tpl/images/0385/0648/fecfa.png)