

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 10:00
Which sentence about particles in matter is true? a. atoms are present in solids and liquids but not in gases. b. the particles of matter are in constant motion. c. the same kinds of atoms are found in different elements. d. when a solid changes to a liquid, the sizes of the particles change.
Answers: 1

Chemistry, 22.06.2019 22:30
Which one of the following bonds would you expect to be the most polar? a) b–h b) n–h c) p–h d) al–h e) c–h
Answers: 1

Chemistry, 23.06.2019 05:30
The term gas is limited to those substances that exist in the gaseous state at
Answers: 1

Chemistry, 23.06.2019 09:20
Asolution of naoh has a concentration of 25.00% by mass. what mass of naoh is present in 0.250 g of this solution? use the periodic table in the toolbar if needed. 0.0625 g what mass of naoh must be added to the solution to increase the concentration to 30.00% by mass? g
Answers: 2
You know the right answer?
Predict the sign of the entropy change of the system for each of the following reactions.
(a)...
(a)...
Questions



Computers and Technology, 10.12.2020 01:00


History, 10.12.2020 01:00

History, 10.12.2020 01:00



Mathematics, 10.12.2020 01:00

English, 10.12.2020 01:00


Mathematics, 10.12.2020 01:00


Mathematics, 10.12.2020 01:00

Chemistry, 10.12.2020 01:00


Social Studies, 10.12.2020 01:00



Mathematics, 10.12.2020 01:00

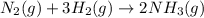
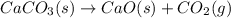
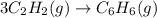
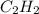 react to give 1 mole of gaseous
react to give 1 mole of gaseous 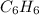 that means randomness become less that means the degree of disorderedness decreases. So, the entropy change will also decreases.
that means randomness become less that means the degree of disorderedness decreases. So, the entropy change will also decreases.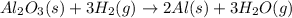
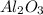 react to give 2 moles of solid aluminium that means randomness become more that means the degree of disorderedness increases. So, the entropy change will also increases.
react to give 2 moles of solid aluminium that means randomness become more that means the degree of disorderedness increases. So, the entropy change will also increases.

