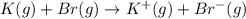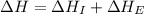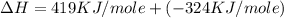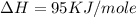Chemistry, 21.11.2019 21:31 shongmadi77
Calculate the energy change for the reaction k(g) + br(g) → k +(g) + br – (g) given the following ionization energy (ie) and electron affinity (ea) values (hint: should one be negative for the reaction? ) ie ea k: 419 kj/mol 48 kj/mol br: 1140 kj/mol 324 kj/mol

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 20:40
If equal masses of the listed metals were collected , which would have a greatest volume ? a. aluminum 2.70,b.zinc7.14,c.copper 8.92,d.lead 11.34
Answers: 2

Chemistry, 21.06.2019 23:40
If the atomic mass of an atom is 34 and the atom contains 13 protons, how many neutrons does the atom contain?
Answers: 2

Chemistry, 23.06.2019 03:20
What kind of intermolecular forces act between a hydrogen fluoride molecule and a hydrogen peroxide molecule? note: if there is more than one type of intermolecular force that acts, be sure to list them all, with a comma between the name of each force.
Answers: 1

Chemistry, 23.06.2019 12:10
What is the correct name for hg(no3)2? mercury (i) nitrate mercury (ii) nitrate mercury nitroxide mercury dinitride
Answers: 1
You know the right answer?
Calculate the energy change for the reaction k(g) + br(g) → k +(g) + br – (g) given the following io...
Questions

Mathematics, 07.01.2020 04:31

Mathematics, 07.01.2020 04:31


Mathematics, 07.01.2020 04:31


Social Studies, 07.01.2020 04:31




Computers and Technology, 07.01.2020 04:31


History, 07.01.2020 04:31

Mathematics, 07.01.2020 04:31






Computers and Technology, 07.01.2020 04:31

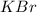 :
: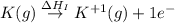
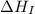 = ionization energy of potassium = 419 kJ/mol
= ionization energy of potassium = 419 kJ/mol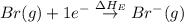
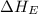 = electron affinity energy of bromine = -324 kJ/mol
= electron affinity energy of bromine = -324 kJ/mol