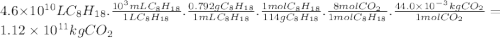Chemistry, 21.11.2019 20:31 jacksonyodell4184
Assuming that gasoline is 100% isooctane, that isooctane burns to produce only co2 and h2o, and that the density of isooctane is 0.792 g/ml, what mass of co2 (in kilograms) is produced each year by the annual u. s. gasoline consumption of 4.6×1010l?

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 05:30
Arecipe calls for 1.2 cups of oil. how many liters of oil is this?
Answers: 2

Chemistry, 22.06.2019 09:30
What are scientists who study fossils called? ( a ) astronomers. ( b ) biologists. ( c ) geologists. ( d ) paleontologists.
Answers: 2

Chemistry, 22.06.2019 12:00
Ageochemist examines a piece of metal that he found in the soil. he performs tests to identify the metal from its density, electrical conductivity, and melting point. which statement best describes his investigation? a. he is determining physical properties that are sufficient to identify the metal.b. he is determining chemical properties that are sufficient to identify the metal.c. he is determining physical properties that are insufficient to identify the metal.d. he is determining chemical properties that are insufficient to identify the metal.
Answers: 3

You know the right answer?
Assuming that gasoline is 100% isooctane, that isooctane burns to produce only co2 and h2o, and that...
Questions




Mathematics, 13.07.2019 08:40

Mathematics, 13.07.2019 08:40


Biology, 13.07.2019 08:40



History, 13.07.2019 08:40



Mathematics, 13.07.2019 08:40

Mathematics, 13.07.2019 08:40


Social Studies, 13.07.2019 08:40

Mathematics, 13.07.2019 08:40

Mathematics, 13.07.2019 08:40

History, 13.07.2019 08:40