
Chemistry, 21.11.2019 20:31 ilovewaffles70
Which statement best explains why the polarity of a h2o molecule differs from the polarity of a co2 molecule? the bond strength is greater in co2. oxygen is more electronegative than carbon. the central atom in h2o has lone pair electrons and the central atom in co2 does not.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 00:40
Which is a difference between molecular compounds and ionic compounds? select the correct answer below: question 5 options: molecular compounds typically form between a metal and a nonmetal, while ionic compounds typically form between nonmetals. molecular compounds result from the transfer of electrons between atoms to form ions, while ionic compounds result from the sharing of electrons between neutral atoms. molecular compounds are formed of discrete, neutral molecules, while ionic compounds are formed of large repeating arrays of opposite charges. molecular compounds have high melting points and high boiling points, while ionic
Answers: 3

Chemistry, 22.06.2019 14:30
Chemistry worksheet - i am not sure what they are asking for exactly?
Answers: 1

Chemistry, 22.06.2019 20:00
What is the molar mass of the anhydrous compound? answer using four significant figures. 36.02 g/mol 120.15 g/mol 156.12 g/mol
Answers: 1

Chemistry, 22.06.2019 20:00
Which of the following would not diffuse through the plasma membrane by means of simple diffusion? 1 oxygen 2 glucose 3 a steroid hormone 4 a lipid soluble vitamin
Answers: 3
You know the right answer?
Which statement best explains why the polarity of a h2o molecule differs from the polarity of a co2...
Questions


Chemistry, 12.10.2020 20:01

Mathematics, 12.10.2020 20:01




Mathematics, 12.10.2020 20:01



Mathematics, 12.10.2020 20:01


English, 12.10.2020 20:01

Physics, 12.10.2020 20:01



History, 12.10.2020 20:01

Mathematics, 12.10.2020 20:01


Mathematics, 12.10.2020 20:01

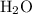 molecule differs from the polarity of
molecule differs from the polarity of 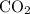 molecule, because Oxygen is more electronegative than carbon.
molecule, because Oxygen is more electronegative than carbon.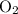 is 3.44.
is 3.44.

