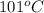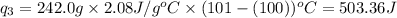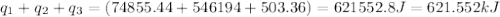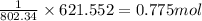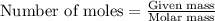Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 18:00
Temperature and kinetic energy are proportional. a) adirectly b) directly c) indirectly
Answers: 2

Chemistry, 21.06.2019 21:30
Balance this equation co2(g) + h2o (g) show that the balanced equation obeys the law if conversation of mass
Answers: 1

Chemistry, 22.06.2019 12:30
Avariable that is not being directly tested during an experiment should be
Answers: 1

Chemistry, 22.06.2019 12:40
In the following table, all the columns for the element calcium are filled out correctly. element electron structure of atom electron structure of ion net ionic charge calcium 1s22s22p63s23p64s2 1s32s22p63s23p64s1 +1 true false
Answers: 2
You know the right answer?
Calculate the mass of methane that must be burned to provide enough heat to convert 242.0 g of water...
Questions




History, 03.08.2019 14:00

Spanish, 03.08.2019 14:00

English, 03.08.2019 14:00

Spanish, 03.08.2019 14:00


History, 03.08.2019 14:00

Mathematics, 03.08.2019 14:00



History, 03.08.2019 14:00




Biology, 03.08.2019 14:00



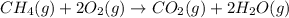
![\Delta H^o_{rxn}=[(1\times \Delta H^o_f_{(CO_2(g))})+(2\times \Delta H^o_f_{(H_2O(g))})]-[(1\times \Delta H^o_f_{(CH_4(g))})+(2\times \Delta H^o_f_{(O_2(g))})]](/tpl/images/0384/9002/f75b3.png)
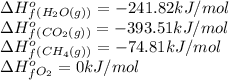
![\Delta H^o_{rxn}=[(1\times (-393.51))+(2\times (-241.82))]-[(1\times (-74.81))+(2\times (0))]\\\\\Delta H^o_{rxn}=-802.34kJ](/tpl/images/0384/9002/75dce.png)
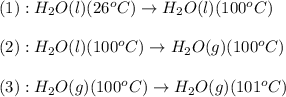
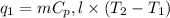
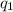 = amount of heat absorbed = ?
= amount of heat absorbed = ?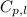 = specific heat of water = 4.18 J/g°C
= specific heat of water = 4.18 J/g°C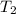 = final temperature =
= final temperature = 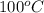
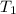 = initial temperature =
= initial temperature = 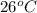
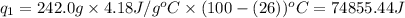
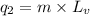
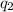 = amount of heat absorbed = ?
= amount of heat absorbed = ?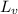 = latent heat of vaporization = 2257 J/g
= latent heat of vaporization = 2257 J/g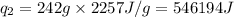
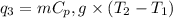
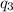 = amount of heat absorbed = ?
= amount of heat absorbed = ?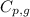 = specific heat of steam = 2.08 J/g°C
= specific heat of steam = 2.08 J/g°C