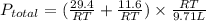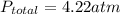The stopcock connecting a 3.06 l bulb containing methane gas at a pressure of 9.61 atm, and a 6.65 l bulb containing oxygen gas at a pressure of 1.75 atm, is opened and the gases are allowed to mix. assuming that the temperature remains constant, the final pressure in the system is atm.

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 18:00
Write a net ionic equation to show that hydrofluoric acid, behaves as an acid in water.
Answers: 1

Chemistry, 21.06.2019 18:20
Which statement accurately describes the relationship between air pressure, air density, or altitude? as altitude increases, pressure increases.as altitude increases, air density increases.air pressure and density are lowest at sea level.denser air exerts more pressure than less dense air.
Answers: 2

Chemistry, 22.06.2019 17:10
Some liquids can be distilled, but only at temperatures that are so high that it is impractical, or so high the compound decomposes. explain why distillation such compounds at significantly less than atmospheric pressure (some degree of vacuum) would solve this problem.
Answers: 2

Chemistry, 22.06.2019 18:30
Which sample at stp has the same number of atoms as 18 liters of ne at stp
Answers: 1
You know the right answer?
The stopcock connecting a 3.06 l bulb containing methane gas at a pressure of 9.61 atm, and a 6.65 l...
Questions



Mathematics, 07.07.2019 17:00

Geography, 07.07.2019 17:00



Social Studies, 07.07.2019 17:00

Mathematics, 07.07.2019 17:00

Mathematics, 07.07.2019 17:00


History, 07.07.2019 17:00









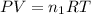
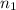 = number of moles of methane gas = ?
= number of moles of methane gas = ?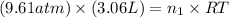
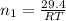
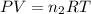
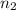 = number of moles of oxygen gas = ?
= number of moles of oxygen gas = ?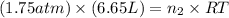
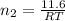
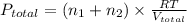
 = final pressure of gas = ?
= final pressure of gas = ?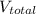 = final volume of gas = (3.06 + 6.65)L = 9.71 L
= final volume of gas = (3.06 + 6.65)L = 9.71 L