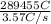Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 04:00
Asample of aluminum foil contains 8.60 × 1023 atoms. what is the mass of the foil?
Answers: 1

Chemistry, 22.06.2019 14:20
You have a liquid that exhibits diltancy. you want to pour it from a bottle. what should you do to the bottle before pouring
Answers: 1

Chemistry, 22.06.2019 18:50
Asample of tin (ii) chloride has a mass of 0.49 g. after heating, it has a mass of 0.41 g. what is the percent by mass of water in the hydrate? %
Answers: 1

Chemistry, 23.06.2019 01:00
Which of the following is in the lanthanide family? a) uranium b) promethium c) silver d) gold
Answers: 2
You know the right answer?
How long would it take to reduce 1 mole of each of the following ions using the current indicated?...
Questions

History, 18.03.2021 20:20

History, 18.03.2021 20:20

Mathematics, 18.03.2021 20:20

Chemistry, 18.03.2021 20:20





Mathematics, 18.03.2021 20:20


History, 18.03.2021 20:20






History, 18.03.2021 20:20

Mathematics, 18.03.2021 20:20

Chemistry, 18.03.2021 20:20

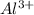 value of current is 1.234 A.
value of current is 1.234 A.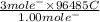
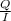
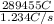
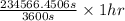
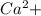 , value of current is 22.2 A.
, value of current is 22.2 A.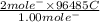
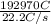
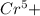 , value of current is 37.45 A.
, value of current is 37.45 A.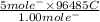
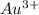 , value of current is 3.57 A.
, value of current is 3.57 A.