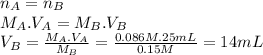Lactic acid (hc3h503) is a monoprotic acid with a k, value of 1.4 x 104 (a) what volume of 0.15 m koh would need to be added to 25 ml of 0.086 m lactic acid to reach the equivalence point? (keep 2 significant figures) ml (b) at the equivalence point, would the aqueous solution be acidic, basic, or neutral? explain why at the equivalence point, the solution will be .. at this stage, all of the lactic acid in the solution will have reacted with the koh added, producing lactate lons (c3h503") and potassium ions (*) in the solution. the potassium ions will not affect the ph, but the lactate ions will make the solution -

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 09:30
Based on its chemical properties, identify the position of each chemical family on the periodic table.
Answers: 3

Chemistry, 22.06.2019 19:00
How many moles of cu are needed to react with 5.8 moles of agno3? cu + 2 agno3 → cu(no3)2 + 2 ag
Answers: 3

Chemistry, 22.06.2019 19:20
15. which of the following is not human-caused groundwater pollution? a. water in an aquifer dissolves elements such as arsenic and mercury from surrounding rock. b. water in an aquifer is contaminated by leachate that seeps into the ground from a landfill. c. water in an aquifer becomes polluted with chemicals used in hydraulic fracturing, or fracking. d. water in an aquifer absorbs harmful bacteria from the drainage field of a septic tank.
Answers: 1

You know the right answer?
Lactic acid (hc3h503) is a monoprotic acid with a k, value of 1.4 x 104 (a) what volume of 0.15 m ko...
Questions

Mathematics, 26.06.2020 17:01


English, 26.06.2020 17:01

Mathematics, 26.06.2020 17:01

Mathematics, 26.06.2020 17:01


Mathematics, 26.06.2020 17:01

English, 26.06.2020 17:01

English, 26.06.2020 17:01

Health, 26.06.2020 17:01

Mathematics, 26.06.2020 17:01

Mathematics, 26.06.2020 17:01

Social Studies, 26.06.2020 17:01

Mathematics, 26.06.2020 17:01

Mathematics, 26.06.2020 17:01



Law, 26.06.2020 17:01


History, 26.06.2020 17:01