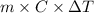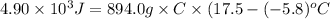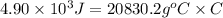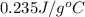Chemistry, 21.11.2019 04:31 crosales102
Achemist carefully measures the amount of heat needed to raise the temperature of a sample of a pure substance from to . the experiment shows that of heat are needed. what can the chemist report for the specific heat capacity of the substance? be sure your answer has the correct number of significant digits.

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 21:30
Calculate the h3o+ concentration in a solution of acetic acid if the concentration of molecular acetic acid present at equilibrium is 9.97x10^-3 m and k for the dissociation is 1.86x10^-5. ch3cooh(aq)+h2o(> h3o^+(aq)+ch3coo^-(aq)
Answers: 2

Chemistry, 22.06.2019 08:30
Which common material is an example of a polymer? (25 pts) a. steel b. plastic c. petroleum d. rubbing alcohol
Answers: 2


Chemistry, 22.06.2019 11:50
Acompound has a molecular weight of 12.124 atomic mass units and the empirical formula c3h40. what is the molecular formula of the compound?
Answers: 3
You know the right answer?
Achemist carefully measures the amount of heat needed to raise the temperature of a sample of a pure...
Questions

Mathematics, 02.11.2020 21:50


Mathematics, 02.11.2020 21:50

Mathematics, 02.11.2020 21:50



Biology, 02.11.2020 21:50


English, 02.11.2020 21:50

Social Studies, 02.11.2020 21:50


Mathematics, 02.11.2020 21:50

Physics, 02.11.2020 21:50


Mathematics, 02.11.2020 21:50



English, 02.11.2020 21:50

Arts, 02.11.2020 21:50

Chemistry, 02.11.2020 21:50

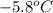 to
to 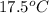 and heat needed is 4.90 kJ (or 4900 J, as 1 kJ = 1000 J).
and heat needed is 4.90 kJ (or 4900 J, as 1 kJ = 1000 J).