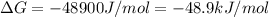Chemistry, 21.11.2019 04:31 alexreddin3127
Acritical reaction in the production of energy to do work or drive chemical reactions in biological systems is the hydrolysis of adenosine triphosphate, atp, to adenosine diphosphate, adp, as described by
atp (aq)+ h20 (l) -> adp (aq) + hpo4 (negative two overall charge) (aq).
for which ? g�rxn = �30.5 kj/mol at 37.0 �c and ph 7.0. calculate the value of ? grxn in a biological cell in which [atp] = 5.0 mm, [adp] = 0.80 mm, and [hpo42�] = 5.0 mm.
a. what is the delta g rxn in kj/mol?
b. is the hydrolysis of atp spontaneous under these conditions?

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 21:10
Harvey mixes two liquids. which observation of the new mixture most likely indicates a precipitate is forming?
Answers: 2


Chemistry, 23.06.2019 05:00
Match each term to its description. match term definition excess reactant a) reactant that can produce a lesser amount of the product limiting reactant b) amount of product predicted to be produced by the given reactants theoretical yield c) reactant that can produce more of the product
Answers: 3

Chemistry, 23.06.2019 05:30
Stoichiometry- i need with 14 and 15! an explanation would be appreciated!
Answers: 1
You know the right answer?
Acritical reaction in the production of energy to do work or drive chemical reactions in biological...
Questions

Biology, 05.05.2020 18:21

Mathematics, 05.05.2020 18:21

Mathematics, 05.05.2020 18:21

Mathematics, 05.05.2020 18:21

Social Studies, 05.05.2020 18:21

Social Studies, 05.05.2020 18:21

Mathematics, 05.05.2020 18:21

Chemistry, 05.05.2020 18:21

Mathematics, 05.05.2020 18:21


Mathematics, 05.05.2020 18:21

Mathematics, 05.05.2020 18:21

History, 05.05.2020 18:21




Mathematics, 05.05.2020 18:21



Mathematics, 05.05.2020 18:21

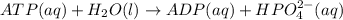
![[HPO_4^{2-}] = 5.0 mM=0.005 M](/tpl/images/0384/0544/f1ef4.png)
![Q=\frac{[ADP][HPO_4^{2-}]}{[ATP]}=\frac{0.0008 M\times 0.005 M}{0.005 M}=0.0008](/tpl/images/0384/0544/0fdb9.png)
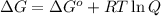
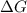 = Gibbs free energy at given conditions
= Gibbs free energy at given conditions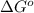 = Gibbs free energy at equilibrium=-30.5 kJ/mol
= Gibbs free energy at equilibrium=-30.5 kJ/mol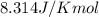
![37^oC=[273.15+37]K=310.15 K](/tpl/images/0384/0544/5ce7f.png)
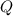 = reaction quotient at 37°C = 0.0008
= reaction quotient at 37°C = 0.0008![\Delta G=-30500 J/mol+(8.314J/Kmol)\times 310.15 K\times \ln [0.0008]](/tpl/images/0384/0544/d6332.png)