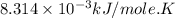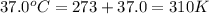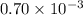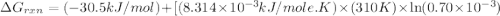Chemistry, 21.11.2019 04:31 juju323261
Acritical reaction in the production of energy to do work or drive chemical reactions in biological systems is the hydrolysis of adenosine triphosphate, atp, to adenosine diphosphate, adp, as described by the reaction atp(aq)+h2o(l)⟶adp(aq)+hpo2−4(aq) for which δ∘rxn=−30.5 kj/mol at 37.0 °c and ph 7.0. calculate the value of δ in a biological cell in which [atp]=5.0 mm, [adp]=0.70 mm, and [hpo2−4]=5.0 mm.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 02:30
Asa choose the correct set of reaction coefficients to properly balance the following chemical equation according to the law of conservation of mass: __s8 + __o2 ==> __so2 1, 1, 8 1, 8, 1 1, 8, 8 8, 1, 1
Answers: 1

Chemistry, 22.06.2019 08:00
Will give ! what are the advantages and disadvantages of nuclear power? check all that apply. one advantage of nuclear energy is that it does not produce carbon dioxide emissions. storage of nuclear waste is a short-term problem associated with nuclear energy. the problem with uranium mining is that a large quantity of uranium must be extracted to meet energy needs because the energy release from uranium fission is so low. safe operation of a nuclear power plant can be jeopardized by a human mistake.
Answers: 1


You know the right answer?
Acritical reaction in the production of energy to do work or drive chemical reactions in biological...
Questions





Mathematics, 06.04.2020 22:23

Mathematics, 06.04.2020 22:23




Mathematics, 06.04.2020 22:23

Mathematics, 06.04.2020 22:23


Chemistry, 06.04.2020 22:23







History, 06.04.2020 22:23

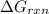 is -49.2 kJ/mol
is -49.2 kJ/mol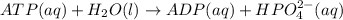
![Q=\frac{[ADP][HPO_4^{2-}]}{[ATP]}](/tpl/images/0384/0553/ccdf0.png)
![[ATP]](/tpl/images/0384/0553/bda18.png) = 5.0 mM
= 5.0 mM![[ADP]](/tpl/images/0384/0553/68360.png) = 0.70 mM
= 0.70 mM![[HPO_4^{2-}]](/tpl/images/0384/0553/c0ca9.png) = 5.0 mM
= 5.0 mM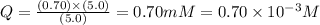
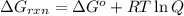 ............(1)
............(1)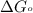 = standard Gibbs free energy = -30.5 kJ/mol
= standard Gibbs free energy = -30.5 kJ/mol