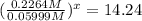At 593k a particular decomposition’s rate constant had a value of 5.21×10−4 and at 673k the same reaction’s rate constant was 7.42×10−3. it was noticed that when the reactant’s initial concentration was 0.2264 m (with a 593k reaction temperature), the initial reaction rate was identical to the initial rate when the decomposition was run at 673k with an initial reactant concentration of 0.05999 m. recall that rate laws have the form rate = k [a]x and, showing work, determine the order of the decomposition reaction.

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 14:30
Alculate the concentration of h3o⁺in a solution that contains 5.5 × 10-5m oh⁻at 25°c. identify the solution as acidic, basic, or neutral.a) 1.8 × 10-10m, basicb) 1.8 × 10-10m, acidicc) 5.5 × 10-10m, neutrald) 9.2 × 10-1m, acidice) 9.2 × 10-1m, basic
Answers: 1

Chemistry, 21.06.2019 17:00
Why do sodium and neon have vastly different chemical and physical properties despite having similar atomic masses?
Answers: 2

Chemistry, 22.06.2019 09:50
What are four significant sources of ghgs that come from wostem washington?
Answers: 2

Chemistry, 22.06.2019 21:00
Which of the following is a physical property flammability heat of combustion solubility and toxicity
Answers: 1
You know the right answer?
At 593k a particular decomposition’s rate constant had a value of 5.21×10−4 and at 673k the same rea...
Questions

Mathematics, 04.08.2019 07:30




History, 04.08.2019 07:30


Mathematics, 04.08.2019 07:30

History, 04.08.2019 07:30

Physics, 04.08.2019 07:30


Mathematics, 04.08.2019 07:30


World Languages, 04.08.2019 07:30

Biology, 04.08.2019 07:30

Social Studies, 04.08.2019 07:30

History, 04.08.2019 07:30


Health, 04.08.2019 07:30

Social Studies, 04.08.2019 07:30

Geography, 04.08.2019 07:30

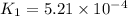
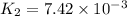
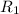
![R_1=K_1\times [A]^x](/tpl/images/0384/0720/5a42c.png)
![R_1=5.21\times 10^{-4}\times [A]^x](/tpl/images/0384/0720/5f894.png) ...[1]
...[1]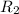
![R_2=K_2\times [A']^x](/tpl/images/0384/0720/6a78b.png)
![R_2=7.42\times 10^{-3}\times [A']^x](/tpl/images/0384/0720/658af.png) ...[2]
...[2]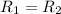 (given)
(given)![5.21\times 10^{-4}\times [A]^x=7.42\times 10^{-3}\times [A']^x](/tpl/images/0384/0720/ebaac.png)
![(\frac{[A]}{[A']})^x=\frac{7.42\times 10^{-3}}{5.21\times 10^{-4}}](/tpl/images/0384/0720/a9fcc.png)