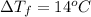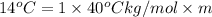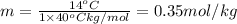Answers: 2


Another question on Chemistry


Chemistry, 22.06.2019 09:30
Which element is the least metallic between cadmium, silver, zinc, or iron?
Answers: 1

Chemistry, 22.06.2019 21:20
40dm3 of gas at 760 torr are heated from 5°c to 50°c what is the new volume
Answers: 3

You know the right answer?
Calculate the molality of isoborneol in the product if, a) the melting point of pure camphor is 179°...
Questions



Mathematics, 18.12.2020 17:00



Mathematics, 18.12.2020 17:00

Biology, 18.12.2020 17:00



Mathematics, 18.12.2020 17:00


Geography, 18.12.2020 17:00




Geography, 18.12.2020 17:00



Mathematics, 18.12.2020 17:00

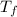 = 165°C
= 165°C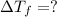
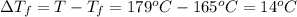
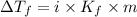
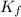 = The freezing point depression constant
= The freezing point depression constant