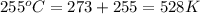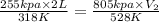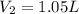Chemistry, 21.01.2020 02:31 dowlingdestiny
Agas at 255 kilopascals and 45°c has an initial volume of 2.00l. the pressure of the gas increases to 805 kilopascals as the temperature is raised to 255°c. what will be the new volume?
1.05 liters
2.28 liters
1.25 liters
0.44 liters
1.50 liters

Answers: 2


Another question on Chemistry



Chemistry, 22.06.2019 15:30
Count the number of each type of atom in the equation below, and then balance the equation. write in the numbers of atoms and coefficients. add a 1 if there should be no coefficient. cs2(l) + o2(g) → co2(g) + so2(g) c [ ] s [ ] o > c [ ] s [ ] o [ ] cs2(l) + [ ] o2(g) > [ ] co2(g) + [ ] so2(g)
Answers: 3

Chemistry, 22.06.2019 17:00
Which statement is true about a catalyst? a: a catalyst decreases the rate of the reaction. b. a catalyst is consumed during a chemical reaction. c. a catalyst lowers the activation energy of a reaction. d. a catalyst increases the reactant concentration during a reaction.
Answers: 1
You know the right answer?
Agas at 255 kilopascals and 45°c has an initial volume of 2.00l. the pressure of the gas increases t...
Questions


Biology, 29.06.2019 08:30



Mathematics, 29.06.2019 08:30

Chemistry, 29.06.2019 08:30

Mathematics, 29.06.2019 08:30

Mathematics, 29.06.2019 08:30







Mathematics, 29.06.2019 08:30




Chemistry, 29.06.2019 08:30

Business, 29.06.2019 08:30

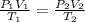
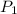 = initial pressure of gas = 255 kpa
= initial pressure of gas = 255 kpa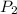 = final pressure of gas = 805 kpa
= final pressure of gas = 805 kpa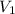 = initial volume of gas = 2 L
= initial volume of gas = 2 L
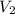 = final volume of gas = ?
= final volume of gas = ?
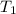 = initial temperature of gas =
= initial temperature of gas = 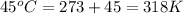
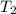 = final temperature of gas =
= final temperature of gas =