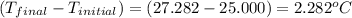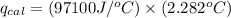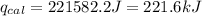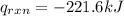Chemistry, 21.11.2019 01:31 Deadpool9609
4.41 g of propane gas (c3h8) is injected into a bomb calorimeter and ignited with excess oxygen, according to the reaction below. the calorimeter (including the water) has a heat capacity of 97.1 kj/°c. c3h8(g) + 5 o2(g) 3 co2(g) + 4 h2o() (a) if the temperature rose from 25.000°c to 27.282°c, what is the heat of the reaction, qrxn?

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 16:30
Energy is released during which phase changes? check all that apply. boiling condensing depositing freezing melting subliming
Answers: 2

Chemistry, 22.06.2019 03:30
Nanotechnology, the field of trying to build ultrasmall structures one atom at a time, has progressed in recent years. one potential application of nanotechnology is the construction of artificial cells. the simplest cells would probably mimic red blood cells, the body's oxygen transporters. for example, nanocontainers, perhaps constructed of carbon, could be pumped full of oxygen and injected into a person's bloodstream. if the person needed additional oxygen-due to a heart attack perhaps, or for the purpose of space travel-these containers could slowly release oxygen into the blood, allowing tissues that would otherwise die to remain alive. suppose that the nanocontainers were cubic and had an edge length of 24 nanometers. part a part complete what is the volume of one nanocontainer? (ignore the thickness of the nanocontainer's wall.) express your answer using two significant figures. v v = 1.4ă—10â’20 l previous answers correct significant figures feedback: your answer 1.3824â‹…10â’20 = 1.382ă—10â’20 l was either rounded differently or used a different number of significant figures than required for this part. if you need this result for any later calculation in this item, keep all the digits and round as the final step before submitting your answer. part b suppose that each nanocontainer could contain pure oxygen pressurized to a density of 81 g/l . how many grams of oxygen could be contained by each nanocontainer?
Answers: 3

Chemistry, 22.06.2019 03:40
Chemical kinetics what was the rate of reaction in trial 3? choose the closest answer.
Answers: 3

Chemistry, 22.06.2019 14:30
Aroom with dimensions 7.00m×8.00m×2.50m is to be filled with pure oxygen at 22.0∘c and 1.00 atm. the molar mass of oxygen is 32.0 g/mol. how many moles noxygen of oxygen are required to fill the room? what is the mass moxygen of this oxygen?
Answers: 1
You know the right answer?
4.41 g of propane gas (c3h8) is injected into a bomb calorimeter and ignited with excess oxygen, acc...
Questions






Mathematics, 13.06.2020 20:57












Mathematics, 13.06.2020 20:57

History, 13.06.2020 20:57

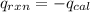
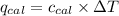
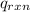 = heat released by the reaction = ?
= heat released by the reaction = ?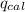 = heat absorbed by the calorimeter
= heat absorbed by the calorimeter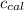 = specific heat of calorimeter =
= specific heat of calorimeter = 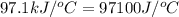
 = change in temperature =
= change in temperature =