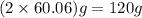Chemistry, 21.11.2019 01:31 Tyrant4life
Which of the following statements describes the correct method of preparation of 1.00 l of a 2.0 m urea solution?
urea = 60.06 g/mol
a) dissolve 120 g of urea in 1.00 kg of distilled water.
b) dissolve 120 g of urea in 880 g of distilled water.
c) dissolve 120 g of urea in enough distilled water to produce 1.00 l of solution.
d) dissolve 120 g of urea in 1.00 liter of distilled water.
e) the density of urea is needed in order to do this calculation

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 18:00
Aballoon inflated with three breaths of air has a volume of 1.7 l. at the same temperature and pressure, what is the volume of the balloon if five more same-sized breaths are added to the balloon?
Answers: 3

Chemistry, 22.06.2019 13:10
Select the correct answer a modure consists of glucose and water. what is the percent composition of glucose in the mixture if it contains 1.3 moles of glucose (cho total mass of the mature is 276 grams? ) and the a 1775
Answers: 1


Chemistry, 23.06.2019 01:00
What type of chemical bond is formed between two atoms of bromine 1. metallic 2. hydrogen 3. ionic 4. covalent
Answers: 1
You know the right answer?
Which of the following statements describes the correct method of preparation of 1.00 l of a 2.0 m u...
Questions



Social Studies, 21.12.2020 19:30

Mathematics, 21.12.2020 19:30


Mathematics, 21.12.2020 19:30



Mathematics, 21.12.2020 19:30

Mathematics, 21.12.2020 19:30


English, 21.12.2020 19:30



Mathematics, 21.12.2020 19:30

Computers and Technology, 21.12.2020 19:30

Mathematics, 21.12.2020 19:30


Mathematics, 21.12.2020 19:30

Advanced Placement (AP), 21.12.2020 19:30