
Hardness in groundwater is due to the presence of metal ions, primarily mg2 and ca2 . hardness is generally reported as ppm caco3 or mmol/l ca2 . to measure water hardness, a sample of groundwater is titrated with edta, a chelating agent, in the presence of the indicator eriochrome black t, symbolized here as in. eriochrome black t, a weaker chelating agent than edta, is red in the presence of ca2 and turns blue when ca2 is removed. redblue ca(in)2+ + edta --> ca(edta)^2+ + in a 50.00-ml sample of groundwater is titrated with 0.0600 m edta. assume that ca2 accounts for all of the hardness in the groundwater. if 12.00 ml of edta is required to titrate the 50.00-ml sample, what is the hardness of the groundwater in molarity and in parts per million of caco3 by mass?

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 22:30
Write the symbol for every chemical element that has atomic number greater than 3 and atomic mass less than 12.0 u.
Answers: 1


Chemistry, 22.06.2019 21:30
Harry lives in a city, and he has a lung condition known as asthma. on certain days, harry has to stay inside because pollutants in the air make it difficult for him to breathe. which of these pollution sources are nonpoint sources that might bother harry if he goes outside? choose the two that apply.
Answers: 3

Chemistry, 23.06.2019 03:00
A0.100-kilogram apple hangs in a tree 1.50 meter above the ground. ignore frictional effects, the total mechanical energy of the apples is
Answers: 1
You know the right answer?
Hardness in groundwater is due to the presence of metal ions, primarily mg2 and ca2 . hardness is ge...
Questions


Mathematics, 10.04.2020 07:23

Geography, 10.04.2020 07:23

History, 10.04.2020 07:23

History, 10.04.2020 07:23

English, 10.04.2020 07:23



English, 10.04.2020 07:24

Mathematics, 10.04.2020 07:24



Arts, 10.04.2020 07:24


History, 10.04.2020 07:24




Biology, 10.04.2020 07:24

Mathematics, 10.04.2020 07:24

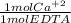 = 7.20x10⁻⁴ mol Ca⁺²
= 7.20x10⁻⁴ mol Ca⁺²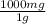 = 72 mg CaCO₃
= 72 mg CaCO₃

