
Chemistry, 21.11.2019 00:31 imogengrzemskip4rq0p
Hz is a weak acid. an aqueous solution of hz is prepared by dissolving 0.020 mol of hz in sufficient water to yield 1.0 l of solution. the ph of the solution was 4.93 at 25.0oc. the ka of hz is a) 1.2×10−5b) 6.9×10−9c) 1.4×10−10d) 9.9×10−2e) 2.8×10−12

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 13:30
What are the chemical names of these compounds? ke: mg3n2: reset next
Answers: 1

Chemistry, 22.06.2019 13:50
What happens when an atom of sulfur combines with two atoms of chlorine to produce sci2? a. each chlorine atom shares a pair of electrons with the sulfur atom. b. an electron is transferred from each chlorine atom to the sulfur atom. c. an electron is transferred from the sulfur atom to each chlorine atom. d. each chlorine atom shares all its valence electrons with the sulfur atom.
Answers: 2

Chemistry, 22.06.2019 17:30
A650 ml sodium bromine solution has a bromide ion concentration of 0.245 m. what is the mass (g) of sodium bromide in solution? a) 103.b)0.00155.c)16400.d) 16.4.e) 0.159
Answers: 2

Chemistry, 22.06.2019 21:00
The rate constant for the reaction below is 6.2 x 10−5 mol l−1 s −1. if the initial concentration of a is 0.0500 m, what is its concentration after 115 s?
Answers: 1
You know the right answer?
Hz is a weak acid. an aqueous solution of hz is prepared by dissolving 0.020 mol of hz in sufficient...
Questions





Geography, 05.06.2020 23:06

History, 05.06.2020 23:06


Mathematics, 05.06.2020 23:06

English, 05.06.2020 23:06








Advanced Placement (AP), 05.06.2020 23:06



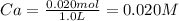
![Ka=\frac{[H^{+}]^{2} }{Ca} =\frac{(1.17 \times 10^{-5})^{2} }{0.020} =6.9 \times 10^{-9}](/tpl/images/0383/6566/93aa1.png)


